Identify the acid-conjugate base pair in this balanced equation: H2SO4 + 2NaOH → 2H2O + - Brainly.com

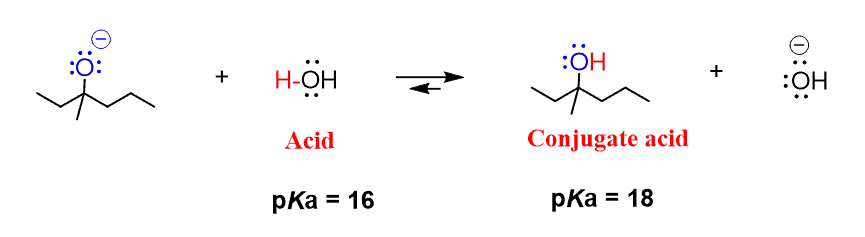
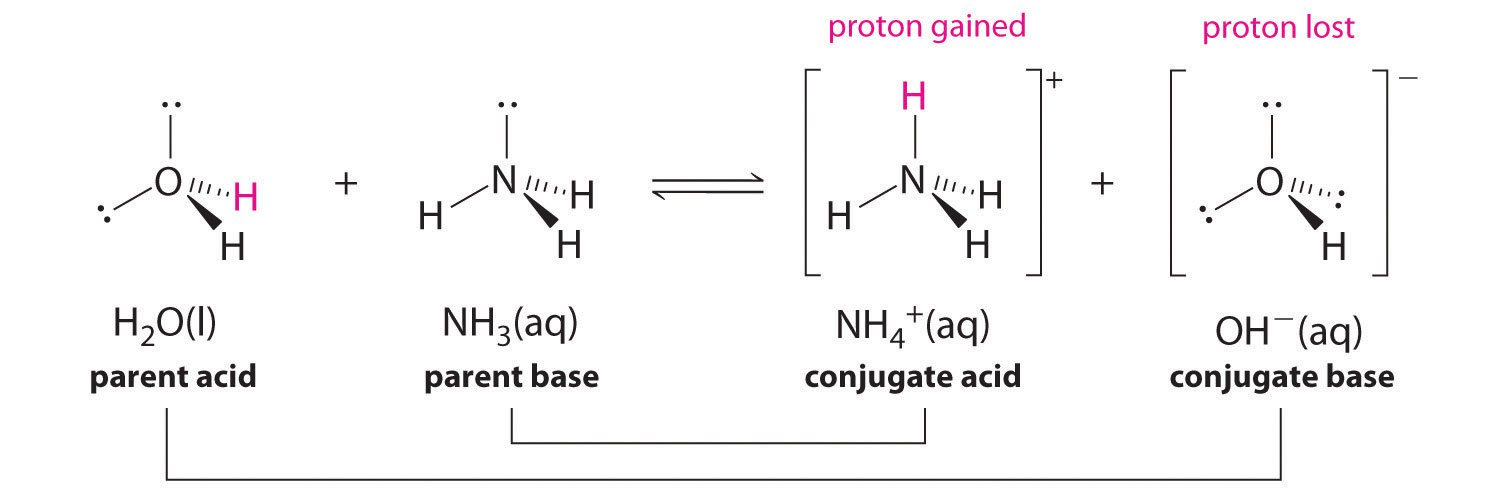
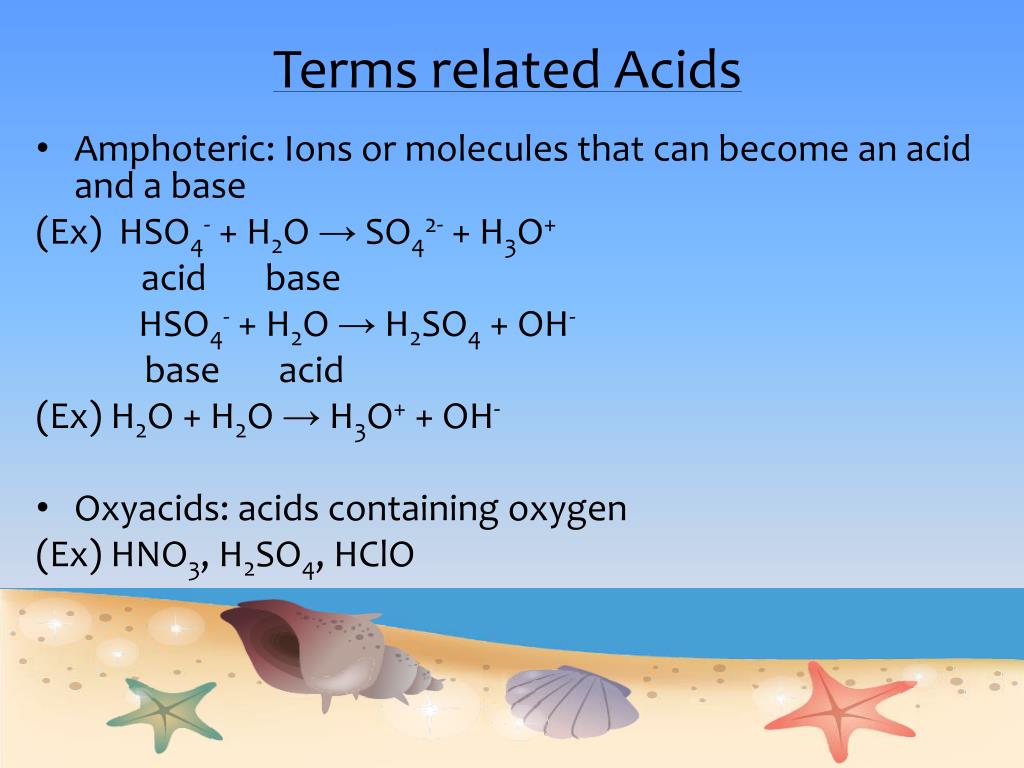
The species: H2O, HCO3^-, HSO4^- and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.

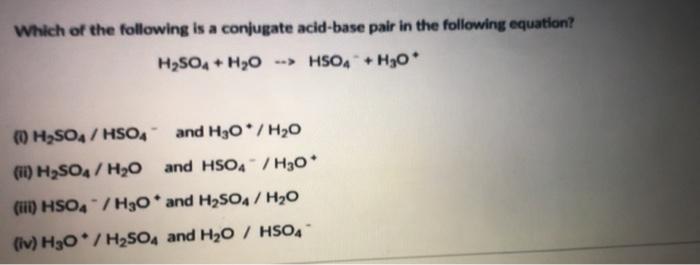
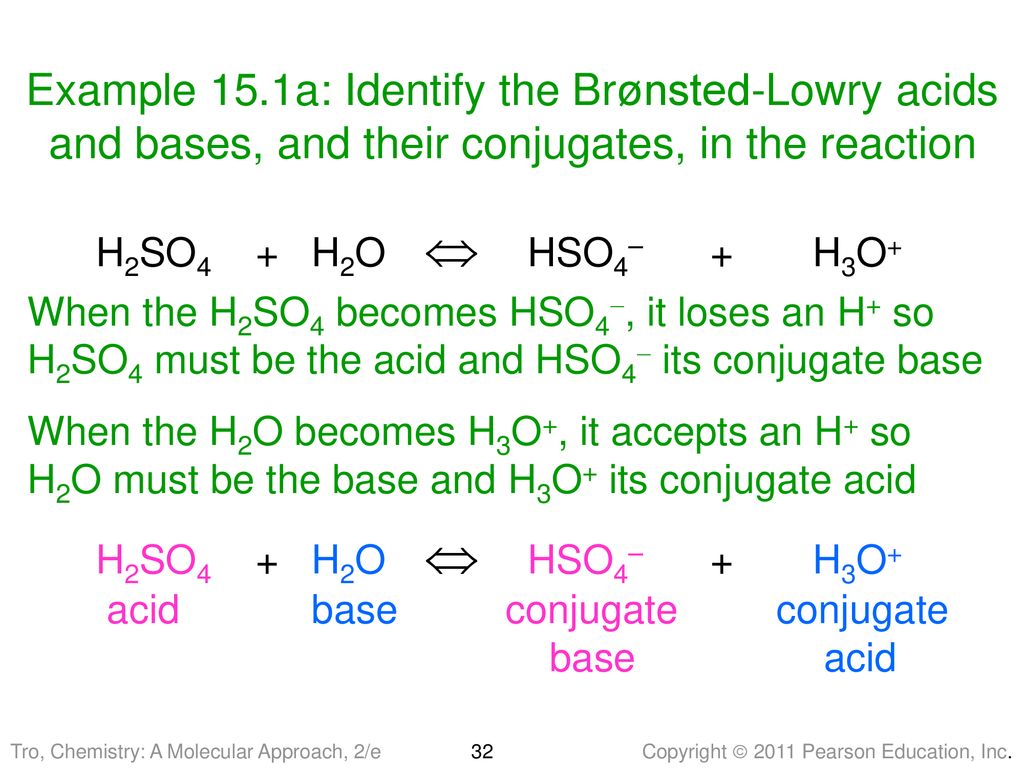
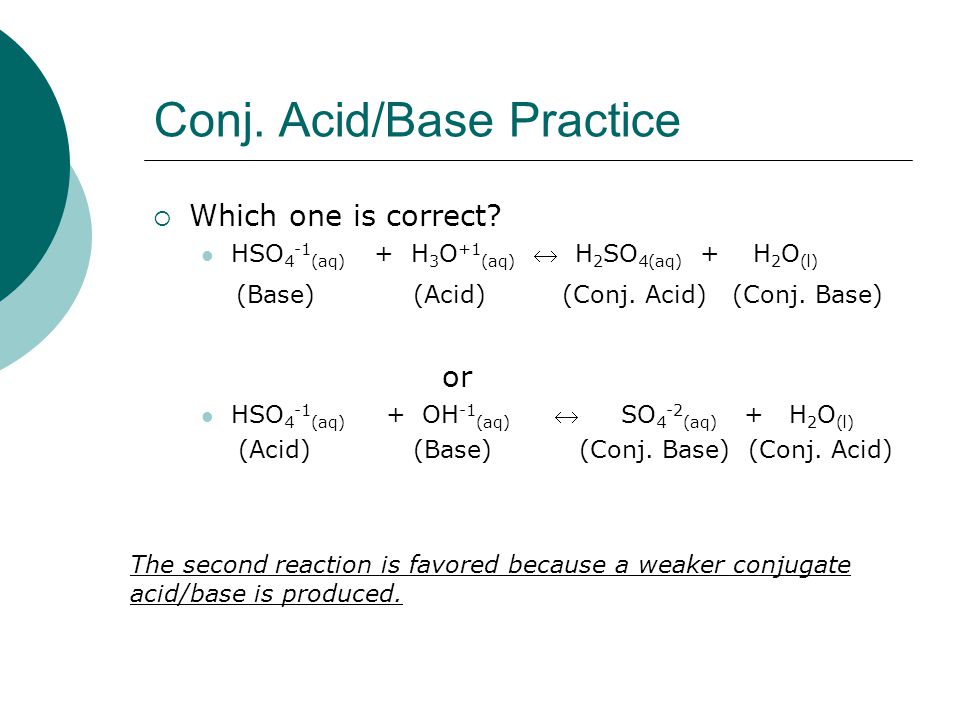
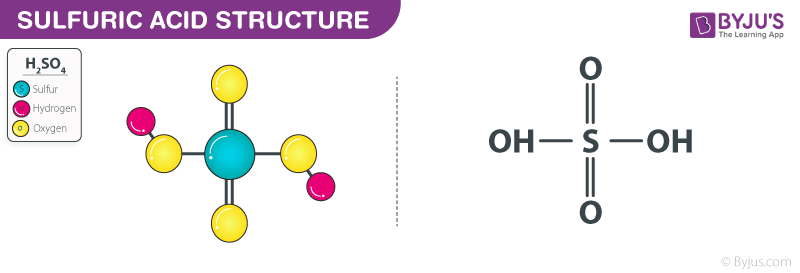
Identify the following reaction, label the acid, conjugate acid, base , and conjugate base: H2SO4 + H2O = HSO4 + H3O+ | Wyzant Ask An Expert

H2O+H2SO4=H3O+SO4 Balance the equation. h2o+h2so4=h3o+so4 water and Sulfuric acid reacts to form - YouTube

What substance is acting as the Brønsted-Lowry acid in the following forward reaction? H2SO4 + H2O - Brainly.com

Identify the conjugate acid-base pairs for the reaction (with the acid written first). CN- + H2O = HCN + OH- |CN- / HCN |HCN / CN- |OH- / H2O |H2O / OH- | Homework.Study.com

Ammonia Catalyzed Formation of Sulfuric Acid in Troposphere: The Curious Case of a Base Promoting Acid Rain | The Journal of Physical Chemistry A
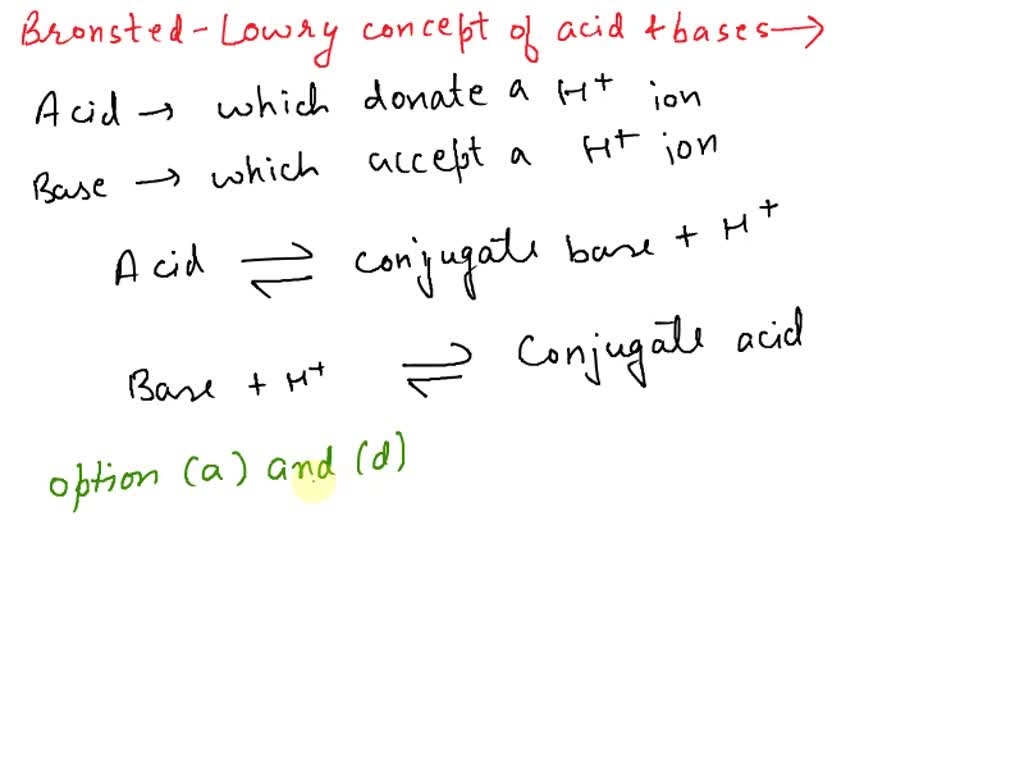
SOLVED: Which of the following represent conjugate acid-base pairs? a. H2O, H3O+ b. OH-, HNO3 c. H2SO4, SO4-2 d. HC2H3O2, C2H3O2-